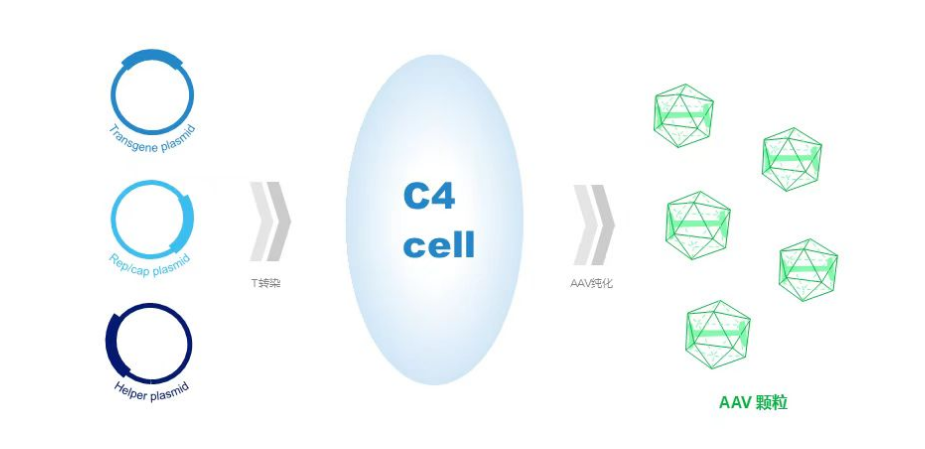
Suspension HEK293 cell
High yield and packaging efficiency for multiple AAV serotype
MCB/WCB were established and identified according to Chp, USP and EU standard
Stability verified in large scale and long term culture
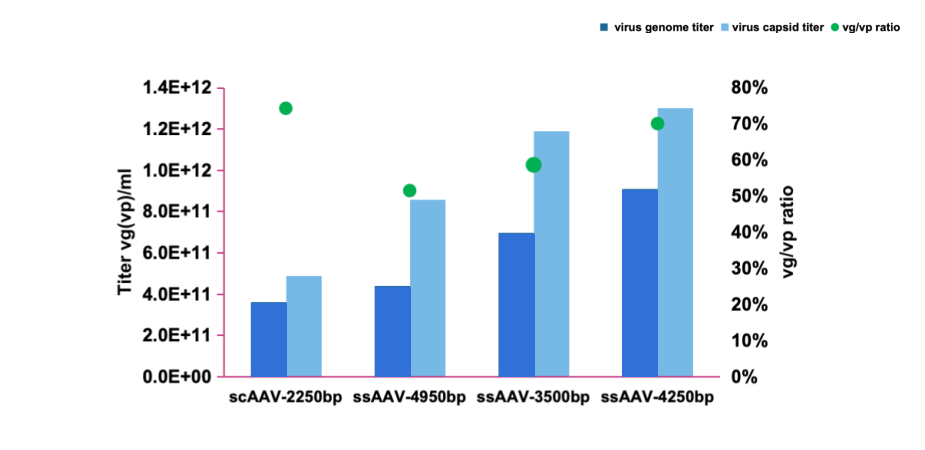
| AAV serotype | Harvest titer by ddPCR |
|---|---|
| AAV2 | 4.00E14 vg/L |
| AAV5 | 1.34E15 vg/L |
| AAV8 | 1.40E15 vg/L |
| AAV9 | 1.20E15 vg/L |
| AAVXL32.1 | 1.6E15 vg/L |