Shanghai, China, November 20, 2025 — Belief BioMed (BBM), an innovative biotechnology company focused on developing cutting-edge gene therapies, today announced that the data from the Phase 1/2 and Phase 3 (NCT05203679) clinical studies and the long-term follow-up data from the investigator-initiated trial (IIT)(NCT04135300) for BBM-H901 (generic name: Dalmacogene Ponparvovec) in adults with hemophilia B, a gene therapy drug developed and manufactured by BBM, have been published in the authoritative international journal Nature Medicine(Impact Factor: 50.0). The paper is titled "Factor IX Padua gene therapy in hemophilia B: phase 1/2 and 3 trials". This study was conducted under the leadership of Professors Lei Zhang and Renchi Yang from the Blood Disease Hospital of the Chinese Academy of Medical Sciences (Institute of Hematology, Chinese Academy of Medical Sciences).

Background
The registration trial of BBM-H901 was a multicenter, single-arm, open-label study consisting of Phase 1/2 and Phase 3. The primary objective of the Phase 1/2 was to evaluate the safety of a single intravenous infusion of BBM-H901 in patients with hemophilia B. A total of 6 1/2participants were enrolled, all receiving a dose of 5×10¹² vg/kg. In Phase 3, 26 participants were enrolled and received the dose determined in the prior Phase1/2 study, with the primary objective to evaluate BBM-H901’s efficacy and safety. In addition, a long-term follow-up study (up to 5 years) was conducted on the coagulation factor IX activity (FIX:C) levels and other clinical data of 10 participants enrolled in the IIT, the results of which were published in The Lancet Haematology in 2022 1.
Results
Phase 1/2 and Phase3
l BBM-H901 exhibited a good safety in clinical trials, with no grade 3-4 adverse events (AEs), serious adverse events (SAEs), or inhibitors observed. The most common adverse reaction was abnormal liver function, characterized by elevated ALT (16.7% in Phase 1/2 and 26.9% in Phase 3) and AST (33.3% in Phase 1/2 and 7.7% in Phase 3) levels, and these participants all recovered following intensified immunosuppressive and liver-protective therapy.
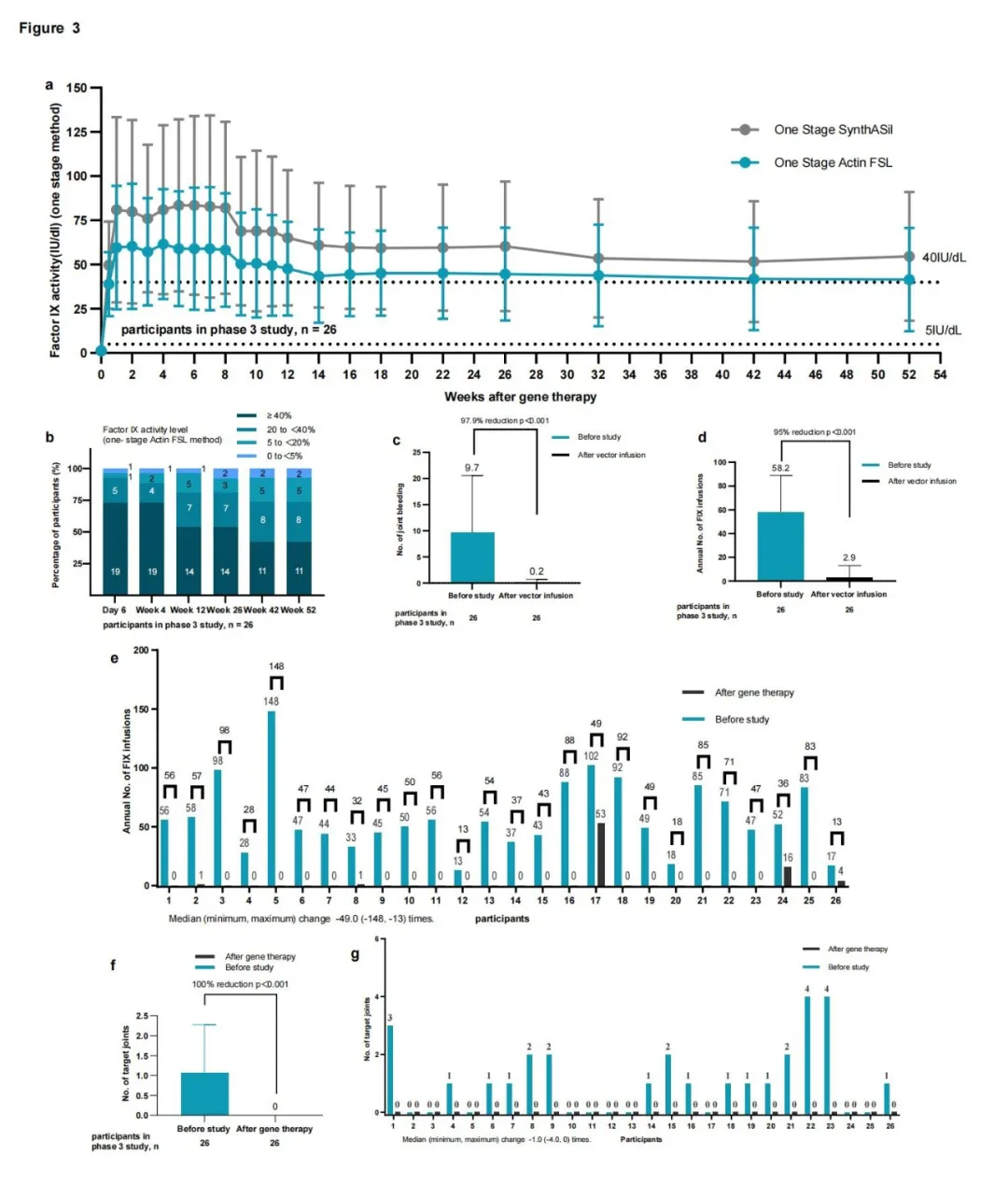
l The results of Phase 3 showed that after 52 weeks follow-up, the mean annualized bleeding rate (ABR) in participants was only 0.6 (95% CI: 0.18-1.99), with a significant decrease compared to the superiority margin, an average ABR of 5.0 for Chinese hemophilia B patients receiving prophylactic treatment. The mean FIX:C level at week 52 after gene therapy was 55.1 IU/dL (SD35.9) (OSA, SynthAsil).
l 3 days after treatment with BBM-H901, the mean FIX:C level in participants reached 67.1 IU/dL (SD23.1), approaching the subsequent peak value. This rapid increase enables participants to quickly eliminate their reliance on traditional coagulation Factor IX (FIX) drug replacement therapy.
l The average number of infusions of coagulation FIX drug decreased from 58.2 times/year (SD 30.67) before gene therapy treatment to 2.9 times/year (SD 10.71) after that.
l The number of joint bleeding frequency significantly decreased after BBM-H901 treatment, and the number of target joints was reduced to zero.
IIT
l Long-term follow-up results of the 10 participants enrolled in IIT showed that, as of the final visit [median time to the last visit was 210 weeks (range: 159-270 weeks)], 70% of the participants maintained FIX:C levels greater than 35 IU/dL, with 40% achieving FIX:C levels above 50 IU/dL (one Stage Assay actin FSL aPTT reagent).
Conclusions
The registration trial of BBM-H901, which involved a larger sample size, systematically confirmed its safety and efficacy in treating adult patients with hemophilia B. The therapy shows a rapid onset of action and sustained efficacy, maintaining stable expression throughout a long observation period of up to 5 years. These findings suggest that BBM-H901 has the potential to provide rapid clinical benefits and long-term, stable therapeutic effects for individuals with hemophilia B.
In April 2025, BBM-H901 was officially approved for marketing by the National Medical Products Administration (NMPA) of China, based on the favorable 52-week results in Phase 3. At the 2025 APSTH Congress held in Wuhan in October 2025, the 2-year FIX:C level results from the registration trial of BBM-H901 were presented, demonstrating that at 104 weeks post-treatment, the mean FIX:C level in 32 participants reached 61.3% (internationally standardized one-stage assay SynthASil reagent), indicating sustained and stable high-level expression and remained comparable to the levels observed at 52 weeks post-treatment2. These results further prove that BBM-H901 has the potential to provide long-term, stable, and high-level protective therapeutic benefits.
The formal publication of the Phase 1/2, Phase 3, and IIT long-term follow-up results of BBM-H901 in Nature Medicine, once again shows Belief BioMed's innovation and R&D capabilities on the global academic stage. Moving forward, Belief BioMed will continue to strengthen innovation and provide more safe and effective treatment options for patients worldwide.
About BBM-H901
BBM-H901 (generic name: Dalnacogene Ponparvovec), has been officially approved by NMPA for the treatment of adult patients with moderate to severe hemophilia B (congenital coagulation factor IX deficiency), and it is also the first approved hemophilia B gene therapy in China. BBM-H901 is developed and manufactured by BBM. It can deliver the optimized human coagulation FIX gene into liver cells of patients to express continuously, and thus the patient's coagulation FIX level is improved and maintained. In 2022, the research results of IIT were successively published in two authoritative international journals, The Lancet-Hematology1 and The New England Journal of Medicine3. In the same year, BBM-H901 obtained the Breakthrough Therapy Designation by the Center for Drug Evaluation, the National Medical Products Administration. In 2024, the long-term follow-up results of more than 3 years of IIT were orally presented at the 2024 International Society on Thrombosis and Haemostasis (ISTH) Congress, and the results of the Phase 3 clinical study were released by BBM at the 66th Annual Meeting of the American Society of Hematology (ASH)4. In 2025, the long-term follow-up results of IIT were orally presented at the 2025 ISTH Congress again5, and the 2-year long-term follow-up results of the registration trial were presented at the Asia Pacific Thrombosis and Haemostasis Conference (APSTH)2. In the same year, the clinical trial results of BBM-H901 were published in the authoritative international journal Nature Medicine6.
References
1. Xue F, et al. Lancet Haematol. 2022 Jul;9(7):e504-e513
2. Xue F, et al. 2025 APSTH. Poster-48.
3. Xue F, et al. N Engl J Med. 2022 Oct 27;387(17):1622-1624
4. Feng X, et al. 2024 ASH. Poster 3582
5. Mankai Ju, et al. 2025 ISTH. OC 69.2
6. Xue F, et al. Nat Med. 2025 Nov
About Belief BioMed
Belief BioMed Inc. (BBM) is a global biotech company that integrates the research and development, manufacturing and clinical application of gene therapy products. The company is committed to providing innovative and more effective gene therapies for severe genetic and chronic diseases through safe and efficient viral vector technology. BBM has developed hundreds of key vector technologies, including HEK293 cell suspension serum-free culture process and full-scale chromatography purification process, and has established a commercial production platform for gene therapy drugs. The company has been building up its capabilities in a variety of fields including novel AAV capsids targeting different tissues, efficient transgene expression cassette design, and advanced clinically applicable vector manufacturing process. It has also established an extensive R&D pipeline covering a wide range of unmet clinical needs in different therapeutic areas such as hemophilia, DMD, Parkinson's disease, osteoarthritis, etc. Several product pipelines have entered clinical studies or submitted IND filings. The Biologics License Application (BLA) of a gene therapy for the treatment of adult patients with hemophilia B, has been approved by the NMPA of China.
Statement
This information is only for the purpose of introducing the company's event and information on that date, and is not intended to promote any company's products and/or services, nor should it be construed as providing any advice or recommendation on the selection of any drugs, medical devices and treatment options.
